Risk Assessment
Risk Assessment
Introduction
This white paper presents a risk assessment process applicable to extractables and leachables (E&L) in systems for packaging, drug delivery, and drug product manufacturing, based on the general principles outlined in ICH Q9, Quality Risk Management. It seeks to demonstrate that the ICH Q9 risk management framework can be applied to the management of leachables, regardless of source, in the drug product lifecycle, with respect to both quality and safety.
Risk assessment constitutes a part of the risk management process and is defined as “the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards.” Risk assessment includes the activities of risk identification, risk analysis and risk evaluation and feeds into the risk control process (Figure 1).
Figure 1. Risk assessment, within the ICH Q9 risk management framework.

According to ICH Q9, risk identification is a systematic use of information to identify hazards referring to the risk or problem; risk analysis is the estimation (scoring) of the risk associated with the identified hazards; and risk evaluation compares the identified and analyzed (scored) risk against a given risk criteria.
As leachables risk evaluation is an important aspect of managing drug product quality and safety, it is appropriate to consider leachables within a risk management framework, and thus how risk assessment can be applied to leachables. The need for leachables-related risk assessment is alluded to in some regulatory guidance, and described in other industry related documents.[1], [2], [3], [4], [5] Leachables risk assessment within the larger risk management framework, however, is not described in current regulatory documentation. In this paper, we will thus:
highlight current gaps in information and understanding related to this topic, particularly where such information could help advance harmonized regulatory approaches to leachables evaluation;
describe concepts and considerations relevant to risk assessment and its elements of risk identification, risk analysis, and risk evaluation pertaining to leachables; and
note existing resources that can assist in providing supporting information on leachables risk assessment.
The scope of our discussion includes drug product packaging, delivery systems, and manufacturing systems relevant to both clinical and commercial production, as well as any modality type, e.g., small molecules, antibodies, cell and gene therapies, oligonucleotides, antibody drug conjugates, and others. Further, although it is recognized that industry uses a variety of risk assessment approaches, and should have the flexibility to do so, we will frame this discussion using some concepts from Failure Mode, Effects and Analysis (FMEA). Using these concepts, the paper discusses a structured examination of how information related to leachables can be assessed with respect to probability, severity, and information hierarchy. Other tools are available for risk assessment as has been demonstrated in the draft chapter USP <1665> and BPOG publications, although these are focused specifically on manufacturing processes.[3] [5]
Gaps
The industry and regulators currently lack a general understanding and agreement on whether there is a regulatory expectation to apply risk management and risk assessment to managing leachables, and if so, how specifically product manufacturers might think about risk assessment as applied to leachables in order to assure product quality and safety. Risk assessment concepts related to leachables, in regulatory guidance, have been very general, as expressed in the FDA’s 1999 packaging guidance risk table, which was later presented in modified form in the USP <1664> (Table 1), and the European Medicines Agency decision tree in its plastic immediate packaging materials guideline (Figure 2). [2] [6] [7]


Figure 2. Decision tree on presentation of documentation for drug product plastic packaging material, from the EMA Guideline on Plastic Immediate Packaging Materials.
Further considerations for risk assessment within a larger risk management approach, for leachables are not provided. Specifically, important gaps in information and understanding related to leachables risk assessment within a risk management framework are:
Currently no agreement on shared glossary of risk terms to apply to extractables and leachables risk assessment (such as found in, e.g., ICH Q9)
No clear risk management guidance for each dose form type (e.g., guidance most clear for high risk dose forms only)
No clear and consistently described process for risk management and, specifically, risk assessment of leachables in any regulatory guidance which is applicable to all sources of leachables (including manufacturing, packaging, and administration) covering all modalities
No agreed definitions for risk factors (Severity, Probability) to allow risk analysis and scoring
No guidance on an appropriate risk evaluation matrix for transformation of risk analysis into risk control decisions
In the following sections, we identify and describe some concepts and principles that could provide a basis for further discussion and creation of a framework for leachables risk assessment, and ultimately a solid basis for guidance on leachables risk management.
Risk Identification
The second step of risk assessment is risk analysis (Figure 1), which includes (i) establishing the factors required to analyze the risk and (ii) developing a scoring system with a description and further advice, in alignment with ICH Q9. Risks identified in step one are scored prior to evaluation, based upon the available knowledge and understanding of severity and probability.
As per ICH Q9, risk is considered to consist of two dimensions severity (hazard) and probability, where,
Severity refers to knowledge and understanding of the material and/or the substances present in it. ICH Q9 defines severity as “a measure of the possible consequences of a hazard.” This hazard is directly related to the safety and quality of the materials and components that are used to manufacture, contain and deliver pharmaceutical products.
Probability is an estimate of the likelihood that the identified risk will occur. Based on factors which relate to knowledge and understanding of the processes that give rise to producing leachables in the drug product or process streams. Various aspects should be taken into consideration when assessing the probability of a risk to occur. The probability of the risk defines the likelihood of the risk event occurring to an extent that it would result in an adverse toxicological or product quality event. The probability of substances leaching during the manufacture, storage and/or administration of a drug product is complex and may including processes which are additive or indeed reductive of leachables (such as clearance steps in a manufacturing process or losses during storage), and is best understood by evaluation of leachables data (or simulation studies).
Scoring can be qualitative or quantitative but should be based on well-defined principles which are science-led and easy to understand and implement (e.g., knowledge of the formulation of a contact material is desirable but not always easy to establish and so makes a poor choice as a principal factor to include in a risk assessment scoring system).
Severity and Probability Information
Examples of types of knowledge that might be gathered by the drug substance and drug product manufacturer to address severity and probability scoring are summarized in Tables 2 and 3. In these tables, note that
The hierarchy of available knowledge for severity and probability is listed in decreasing impact order; and
An exhaustive extractable study may typically be conducted with the intent to gain a qualitatively complete understanding of what substances are present in a given material. As such, this type of extractable study is aligned to severity associated with a material and its component substances.


Further description of information affecting severity or probability can be found in Appendix 1. A set of physicochemical factors inherent to the system determine both the partitioning and diffusion behavior of a chemical with a potential to leach. These factors are addressed in more detail in Appendix 2. The accumulation of leachables in the drug solution as a result of partitioning, solubility limits and diffusivity can be either experimentally determined or calculated based on science-based and validated models for partitioning and diffusion. Some key principles of this type of mathematical modeling (mass transport modeling) approach are discussed in Appendix 3.
Scoring Severity
A scoring system for severity can be developed on the basis of the available information. Below is an example of a four-level scoring system for severity giving a basic definition and example of how the definition might be met when scoring. The numerical scoring approach on a scale from 1-10 is illustrative only, and other scores can be used for a mathematical risk level determination (Table 4). Additionally, different approaches such as a categorization ladder can be applied, e.g., very low, low, medium, high; low, medium, high, severe, if the risk level discrimination mechanism is based on a risk map.

Scoring Probability
As with severity, a scoring system for probability can be developed. This can be done based on knowledge of factors contributing to unacceptable levels of leachables in the drug product. The levels and types of leachables can be estimated based on one or more of the following approaches:
(i) experimental studies (simulation studies, extractables studies, and/or leachables studies
(ii) qualitative consideration of physicochemical factors determining leaching as delineated in Appendix 2, or
(iii) mathematical modeling (mass transport modeling) as discussed in Appendix 3.
Note that items (ii) and (iii) represent tools to check or to complement experimental data, in particular when severity is low or experimental analysis is challenging.
Table 5 presents an example for probability scoring based on four levels with a definition for each level and an example of how the definition might be met. This scoring approach is illustrative only, and other scores can be used as noted above.

The probability terms share many of the same characteristics of the terms described in the draft USP <1665> and the BPOG leachables best practices document. It is important to make use of all available data and knowledge that exists at the time of risk assessment and to weight it accordingly when scoring is done.
The example scoring given in this section leads to a 4 x 4 matrix. Other approaches can be used to derive different matrices (e.g, 3 x 4, 2 x 3, etc.). All would share the common goal of establishing risk by combining severity and probability to derive an overall risk score that can be used for a risk evaluation.
Risk Evaluation
Risk evaluation is closely coupled to risk analysis and is the final step in risk assessment. The matrix developed for risk analysis is used during risk evaluation. Risk evaluation is the process step in which the calculated risk score is evaluated against criteria.
On the basis of the four-level scoring schemes for probability and severity outlined in Section 4, a risk priority number (RPN) can be calculated (the product of severity and probability) and used to create a pre-agreed risk evaluation criterion. The numerical scores and RPN are illustrated in Figure 3 below.
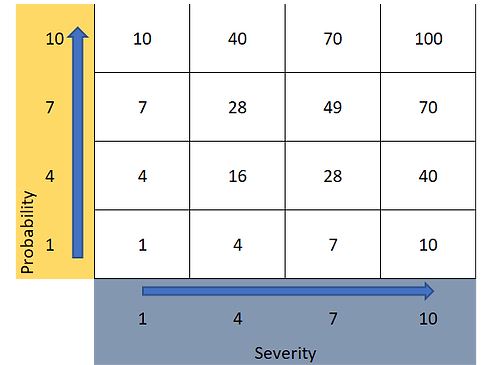
Figure 3: Example RPN matrix for use in risk evaluation.
Risk evaluation criteria can be created to reflect a variety of approaches. For example, a criterion for evaluation might be, risk reduction is required for all RPN greater or equal to a RPN of 28. However, this does not account for scenarios where probably or severity has scores of 10 (a score of 10 representing either almost certain leaching or an unacceptably high hazard). Thus, it may be prudent to modify the criterion to read, RPN scores greater or equal to 28 or individual scores of probability or severity equal to 10 require action. The nature of the action can be tailored to a choice of risk reduction or lead to risk acceptance for low risks.
If the alternative (non-numeric) descriptions of severity or probability are to be used, the process is the same. A clear statement of risk evaluation must be written leading to clear outcomes to pass to risk control.
Regardless of whether numeric or non-numeric scoring is used, it is suggested risk evaluation has the following characteristics:
Scenarios that are low risk have been sufficiently assessed not to require further risk reduction (risk acceptance).
Events that are highly probable or severe consequences require some form of risk reduction. Events which have both high probably and high severity also require risk reduction.
The borderline scenarios (e.g., low likelihood of leaching for a toxic compound) in which an event might be improbable, but have severe consequences, or highly likely, but with little impact, might also require risk reduction, though the outcome is more subjective. The subjectivity of these borderline scenarios are the ones which benefit most from a clear risk evaluation criterion.
As such, a 2-level outcome (Figure 4) or a 3-level outcome (Figure 5) can be generated, depending on the risk evaluation approach taken.
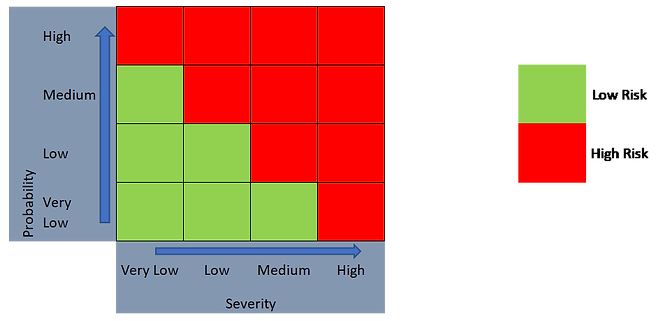
Figure 4. 2-level risk categorization approach using a 4 x 4 risk matrix.
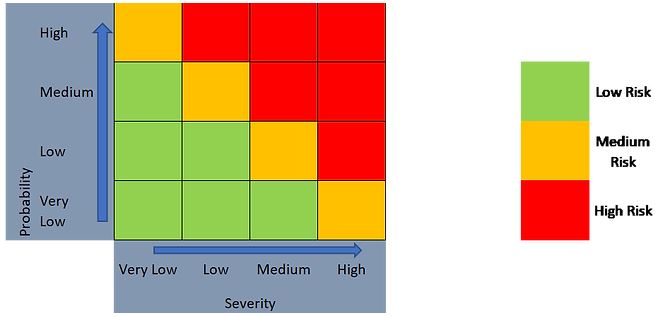
Figure 5. 3-level risk categorization approach using a 4 x 4 risk matrix.
Other risk assessment models might have different scoring definitions, or indeed a different number of outcome levels. But the basic requirements are the same: (1) a method to score risk and (2) a method to evaluate the risk. However, it is recommended that risk evaluation should lead to a clear outcome with regard to risk control. It should be simple to understand and lead to simple risk control choices with clear links to risk acceptance or risk reduction. The concepts of risk acceptance and risk reduction are discussed in more detail in the risk control sections.
After risk assessment is completed the process moves to risk control where the assessed risks are further considered. Lifecycle management may include a return to risk assessment as part of a risk review. The section on lifecycle management gives further details on this.
References
[1] Guideline on process validation for the manufacture of biotechnology-derived active substances and data to be provided in the regulatory submission. Section 6.1.3. European Medicines Agency. 2016
[2] Container Closure Systems for Packaging Human Drugs and Biologics, Guidance for Industry. U.S. Food and Drug Administration. May 1999.
[3] USP <1665> Characterization of Plastic Materials, Components, and Systems Used in the Manufacturing of Pharmaceutical Drug Products and Biopharmaceutical Drug Substances and Products. United States Pharmacopoeia. 2019.
[4] ISO 10993-18:2020 Biological evaluation of medical devices — Part 18: Chemical characterization of medical device materials within a risk management process. International Standards Organization. 2020.
[5] Best Practices Guide for Evaluating Leachables Risk from Polymeric Single-Use Systems Used In Biopharmaceutical Manufacturing. Biophorum Operations Group (BPOG). https://www.biophorum.com/best-practices-guide-for-evaluating-leachables-risk-from-polymeric-single-use-systems-used-in-biopharmaceutical-manufacturing/ Accessed 24 March 2020.
[6] USP <1664> Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery systems
[7] European Medicines Agency. Guideline on Plastic Immediate Packaging Materials. 2005. https://www.ema.europa.eu/en/plastic-primary-packaging-materials
[8] ISO 10993-1:2018. Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process.
[9] USP <87>. Biological Reactivity Tests – In Vitro
[10] USP <88>. Biological Reactivity Tests – In Vivo
[11] USP <660> Containers — Glass
[12] USP <661> Plastic Packaging Systems and Their Materials of Construction
[13] USP <661.1> Plastic Materials of Construction
[14] USP <661.2> Plastic Packaging Systems for Pharmaceutical Use
[15] USP <381> Elastomeric Closures for Injections
[16] USP <232> Elemental Impurities — Limits
[17] Ph.Eur. 3.1 Materials Used for the Manufacture of Containers
[18] Ph. Eur. 3.2 Containers
[19] VDI 2017:2019-07. Medical Grade Plastics (MGP). https://www.beuth.de/en/technical-rule/vdi-2017/307527917
[20] Jenke, D. (2011). “A general assessment of the physicochemical factors that influence leachables accumulation in pharmaceutical drug products and related solutions.” PDA Journal of Pharmaceutical Science and Technology 65(2): 166-176.
[21] Piringer, O. G. B., Lawrence A. (2008). Plastic Packaging – Interaction with Food and Pharmaceuticals, Wiley VCH.


